The operation of a digital camera with nickel-cadmium and nickel-metal hydride alkaline sealed cylindrical rechargeable batteries of size AA prompted me to realize the need to manufacture a device for determining the internal resistance of a battery. In a digital camera, the battery operates at sufficiently high discharge currents - 300 - 600 mA. Practice has determined that the automation of digital cameras incorrectly determines the residual capacity of the battery and turns off the camera. And the batteries removed from the camera still have to be discharged in less finicky devices: in flashlights, toys, and players.
The determination of the internal resistance of the battery, I hope, will give me the opportunity to determine in practice the suitability of a particular battery for use in a digital camera. Advertising in this matter turned out to be a bad clue, if we take into account that the electromotive force of nickel-cadmium batteries is 1.2 volts, and the electromotive force of nickel-metal hydride batteries is 1.25 volts (according to Wikipedia).
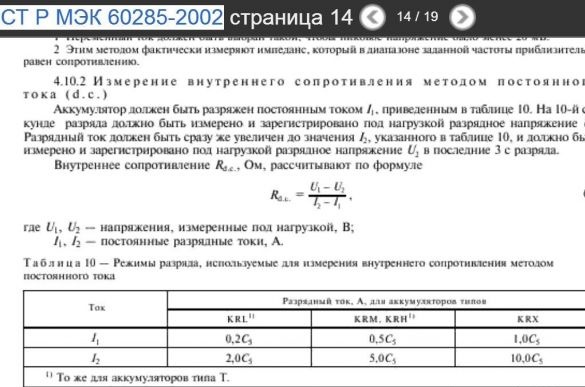
The methodology for measuring the internal resistance of batteries, I mainly used from the document - GOST R IEC 60285-2002 "Sealed cylindrical nickel-cadmium batteries".
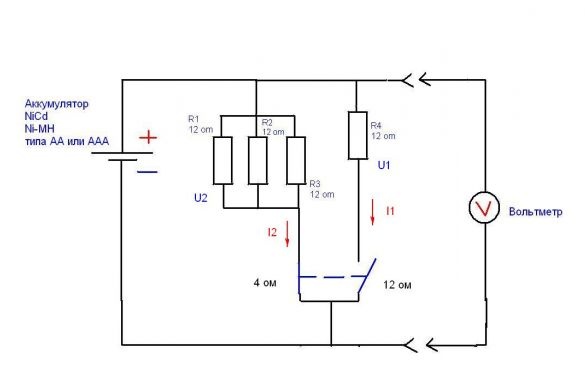
I used a resistance of 12 ohms. I assembled from them and a toggle switch 2 bit circuits. Discharge currents were about 100 mA, 300 mA. To measure the voltage across the resistances, I used the APPA93N multimeter in the 2 Volt range. Assembled a circuit from what was. I did not find less resistance. I used the case from an old microcalculator. I set the resistance on a piece of the breadboard. Empirically found that to assess the quality of power sources it is better to increase discharge currents.
Diagram of the internal resistance meter nickel - cadmium, nickel - metal hydride alkaline sealed cylindrical batteries and alkaline batteries of size AA:
Ready-made internal resistance meter nickel-cadmium, nickel-metal hydride alkaline sealed cylindrical batteries and alkaline batteries, size AA:
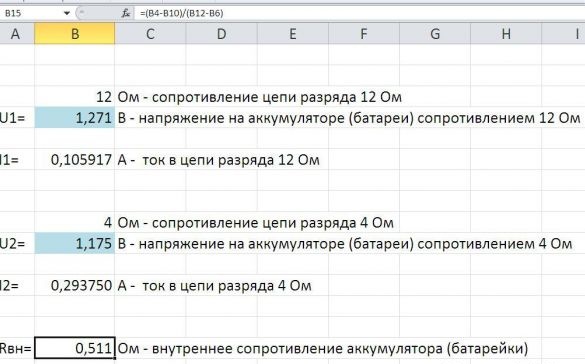
The first test with nickel - alkaline metal hydride sealed cylindrical batteries of type AA Pleomax with a capacity of 2300 mAh. The voltage (U1) on the battery loaded on a 12 ohm resistor was 1.271 Volts. Using Ohm's law, we determine the current strength in the circuit (I1). The current strength is 0.105917 amperes or 105.917 mA. Toggle the toggle switch. The voltage (U2) on the battery loaded on a 4 ohm resistor was 1.175 Volts. Using Ohm's law, we determine the current strength in the circuit (I2). The current strength is 0.29375 amperes or 293.75 mA. Using the formula for determining the internal resistance of a battery from GOST R IEC 60285-2002 “Sealed cylindrical nickel-cadmium batteries” (Uvn = U1-U2 / I2-I1), we calculate it to be 0.511 Ohms. I automated the calculations. To do this, I created the Wicrosoft Exel file - calculations.xlsx.
Calculations.rar
In this file, you can substitute the measured voltage values U1, U2 and your values of load resistances and get the result of the calculation - the internal resistance of the battery or battery.
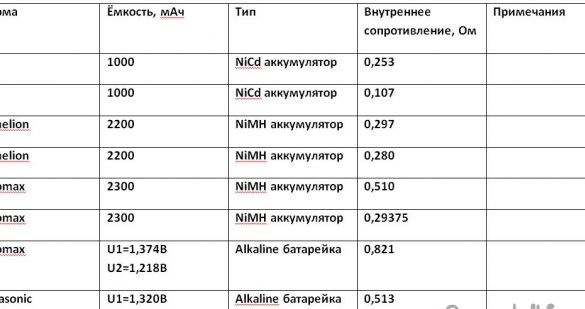
I have accumulated a small amount of batteries. I decided to test them. I entered the test results in the table.
In the process of working on a battery internal resistance meter, I learned that since 2005, nickel - metal - hydride batteries with low self-discharge have appeared on the market. These batteries are sold as “ready to use” or “pre-charged”. A very interesting feature of nickel - metal - hydride batteries with low self-discharge is the significantly lower internal resistance than conventional NiMH batteries. Another feature of these new batteries is their higher cost. And to assess the quality and belonging of a particular battery to the declared one as “ready to use” or “pre-charged”, my battery internal resistance meter will help you.
Good luck