At the beginning of June last year, a hydrogen generator from a fire extinguisher was assembled.
You will learn more about the assembly process by watching the video.
He copes with the generation of hydrogen well, but cannot be used as a gas source for a gas burner. There are two reasons for this. Firstly, there is no normal regulation of gas supply, and secondly, there is a danger of the flame getting directly into the cylinder. The likelihood that this will happen in principle is too vague, but still it cannot be completely ruled out. Therefore, some kind of flame cutting mechanism will be required. All this will be described in today's article. Even in several versions.
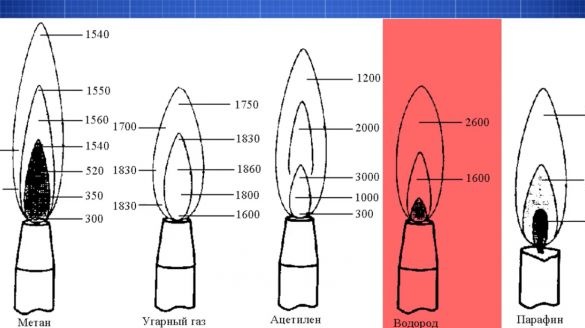
The use of hydrogen as fuel for gas burners is justified. Since the temperature of the hydrogen flame is higher than that of the flame of many other gases. In addition, producing hydrogen is very simple. For the production of hydrogen, aluminum is required in any available form. You will also need alkali. A kilogram of alkali can be bought for less than 100 rubles.
You can get a lot of hydrogen from it. From a kilogram of sodium alkali (caustic soda), 840 liters of hydrogen are obtained. And from a kilogram of potassium alkali, approximately 600 liters of hydrogen are obtained. At the same time, for every 10 liters of hydrogen, only 8 g of aluminum is required. In short, a can of aluminum (20 l) of hydrogen is obtained from one beer can. And this is awesome.
The author decided to adjust the gas supply with a bolt and a pair of nuts. It is necessary to install a bolt on the very edge of the locking and starting device. The farther from the edge, the smoother the adjustment. The bolt needs to be tightened tightly. So that he never got out at all. Just for such purposes, the author has plate-shaped washers with a notch and these are toothy nuts.
In the trigger lever, you need to make such a slot so that the lever does not rest against the bolt.
Next, you need a washer and a lamb. Twisting it, it will be possible to very smoothly regulate the gas supply.
Of course, this will not replace the gearbox, but the gas valve will definitely be able to replace it.
Now we load all aluminum scraps and failed castings, other aluminum containing parts and pieces of foil. In short, everything that was nearby.
Aluminum can be loaded a lot at once. The bigger, the better. But within reasonable limits. Of course, you do not need to stuff to the eyeballs. 100 g of aluminum will be enough.
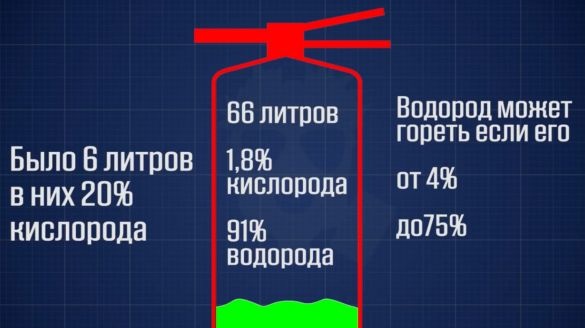
It is easier to regulate the volume of hydrogen produced using alkali.100 g of potassium alkali will produce approximately 60 liters of hydrogen. Given that the fire extinguisher can confidently hold 26 atm, and its free volume is about 6 liters, then it can produce no more than 150 liters of hydrogen at a time. This is very good.
You need to pour 500 grams of water, well, or even a little more. The reaction begins immediately and hydrogen is released.
The gases mix very well. The streams of hot hydrogen and water vapor emanating from the surface of the solution pass through the entire volume of the fire extinguisher. At the same time, they mix all the gases that are there.
Initially, 6 liters of air, which were in the cylinder, contained 20% oxygen. But after 60 liters of hydrogen were produced, the volume of gases increased by more than 10 times. That is, the oxygen content was already only 2%.
If the hydrogen content in the gas mixture is above 75%, then such a mixture is not able to burn without additional oxygen. And as a result, it is not able to detonate. That is, it is absolutely explosion proof. But you should not rely only on this, you need to make some kind of reliable flame cutter. The most affordable, of course, is water. We fix a small water tank on the generator body. We will make 2 holes in its lid, and pass tubes through them.
Fill the tank with water. Now she will create a barrier to a hypothetical flame.
We will try to use a compressed piece of a copper tube as a burner. Hydrogen burns with an almost invisible flame and constantly fades.
This is due to the fact that the pressure is jerky, the volume is too small at the chamber and the flame cut-off mechanism. We’ll increase anything now.
A 5 liter plastic bottle will perfectly smooth out the jerks resulting from bursting bubbles. But it must be purged to expel oxygen from the tank. Have to lose at least 5 liters of hydrogen, but nothing, all this will be fixed a little later.
It burns evenly. There is a slight coloration of the flame due to water vapor coming with hydrogen. Copper wire melts generally easily, and this is already above 1000 ° C however. Even such a simple burner works very well. Of course, it does not pull on a lightsaber, but it already looks like a sharpening Jedi.
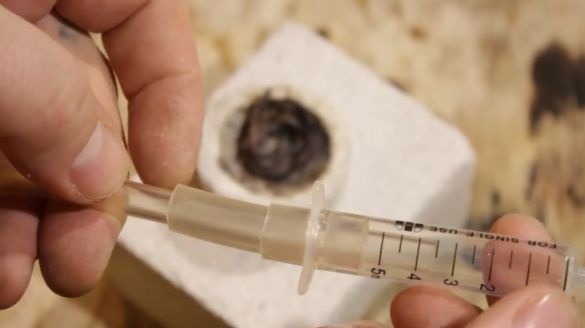
Next, you need syringes of different sizes. They come with needles with different diameters of 1.2 mm, 0.8 mm and 0.7 mm. If you grind off the sharp part to them, we get quite good burners of different capacities. Then the author connected a syringe, on which you can put on different needles.
Small games work a little weak, but the big one burns, already with a whistle.
A syringe burner is very inconvenient. Constantly need to hold all the details so that they do not creep away from high pressure.
Therefore, the author made such a copper burner by drilling a hole with a diameter of 1 mm in the tube.
Let's add a little destruction. Destroy the aluminum can and try to melt a little broken chemical utensils.
In principle, the system works, but the huge gurgling capacity is a little annoying. It is nevertheless necessary to make it somehow more compact. Let's make a flame arrester. Its principle is very simple. But for this you will have to buy a pair of fittings and an extension cable 60 mm long.
Inside it is necessary, as densely as possible, to fill a copper wire. We will use all the useful space even into the fitting we push it.
We will collect threaded connections on paste and tow. It may not be properly sealed by super, but the pressure in this part of the system will not be too large and should not seem to poison. Tightly-tightly we stuff the wire inside to fill the internal volume as evenly as possible. In the end, you can even use a hammer. But despite this, air passes through such an arrester anyway with little or no effort.
We fasten the last spare part. It is necessary to check it somehow. For this, the author repeatedly picks up hydrogen inside this part.On one side it puts cotton wool soaked in acetone. His vapors flare up at the slightest flame.
If the flame can pass through this damper, then the fleece will flash. Please note that the system is not even under pressure. This will be just like the case when the pressure in the cylinder has decreased to a minimum and there is a great danger of flame entering the cylinder. From time to time, the author himself set fire to the cotton wool to check if the acetone vapor had completely evaporated. And if necessary, he moistened it again.
He tried to set fire to one side of the arrester, as well as the other. Even when hydrogen with cotton caught fire from the side of the large nozzle, it was still possible to set fire to the outgoing hydrogen several times from the nozzle of a smaller diameter. This suggests that the hydrogen inside cannot in any way burn normally. It mixes with air and gradually leaves the inside. Hydrogen cannot burn normally, because the wire takes heat from the flame and cools it to room temperature. And hydrogen can ignite at atmospheric pressure only when the temperature is above 500 ° C. If the temperature drops, the combustion reaction decays and generally ceases. In short, this flame arrester stupidly cools the flame, and it does not matter at what concentration hydrogen and oxygen will be supplied. This means that you can screw it to the cell and use it calmly. It's time to screw it to its rightful place.
Now you don’t need to blow anything and waste hydrogen to nothing.
Thank you for attention. See you soon!
Video: