Today we will try to make our own matches, after which we will check how much they will differ from the purchased ones.
But first, a little history. The first similarity of matches appeared in ancient China. But those sources of fire, served only to facilitate the ignition process and were ordinary elemental sulfur, which was smeared on thin slivers. In Europe, matches began to appear only in the 19th century and in their early form were dangerous. That is, they were ignited by friction on any surface, which was dangerous, since they could ignite when rubbing against each other inside the box. The first safe matches appeared only in 1855. The Swedish chemist Johan Lundstrom invented them. In principle, in this form they have survived to our days almost unchanged.
These are exactly the kind of Swedish matches we will do today.
To make them we need:
1. Toothpicks from birch (it is better to use straws from aspen)
2. Skewers for barbecue (for the manufacture of larger matches)
3. Fire retardant (2% solution of ammonium dihydrogen phosphate)
4. Paraffin (paraffin candle)
5. Finely ground sand
6. Sulfur
7. Gelatin (ordinary food)
8. Potassium Dichromate
9. Sodium Alginate
10. Water
11. Potassium Chlorate
12. Iron oxide or other inert dyes (optional)
13. Cardboard (for the manufacture of matchbox)
14. Red phosphorus
15. PVA glue
Making matches begins with the simplest thing - with an ordinary tree. The wooden part of the match is called a straw. It is most often made from aspen, but for lack of it, as a straw, the author will use ordinary birch toothpicks, as well as kebab skewers for larger matches.
The first step in the production of matches is to impregnate the straws with a flame retardant. This is a substance that prevents wood decay. The fact is that after burning wood, coal remains, which continues to smolder and turn into light ashes, which can cause a lot of inconvenience if it gets on clothes or something else.
To avoid trouble when using matches, the straws are impregnated with a two percent solution of ammonium dihydrogen phosphate, that is, an acid salt of ammonium and phosphoric acid.
After impregnation and drying, it is clearly seen that when the straw is burned, the coal formed does not decay, which is very convenient.
The author has quite ancient matches in the collection, which are already more than 100 years old.They were still made in Reval, which is the name of Tallinn in Tsarist time before the revolution of the 17th year. They still burn fine, but due to the lack of flame retardant impregnation, the burned head of the match quickly falls off and continues to smolder, which can cause a fire or even a fire.
So the impregnation of matches today is simply a necessary measure.
Nevertheless, for the further production of matches, the straws must also be saturated with a combustible substance, which will facilitate the burning of the tree and will take on most of the energy. Most often, ordinary paraffin is used for this. To do this, the author melted a paraffin candle and lowered a chopped wooden straw into hot paraffin. It turned out something like deep-fried paraffin and wooden chips.
Interestingly, the smell in this process was really pleasant, since the tree contains sugars, which when roasted give a sweet aroma. However, this is not all. After cooling, the straw soaked in paraffin, the most important thing is to be applied to its tip - the head of the match, which is called sulfur in the common people. The so-called sulfur, is a rather complex mixture, which can consist of 4 or 10 different substances.
For this homemade product, the author took the simplest and most classic recipe. At first he weighed 39% of finely ground sand.
And yes, do not be surprised, sand, playing the role of a flame retardant, simply needs to be added to the mixture for the match head. Otherwise, when ignited, the match will simply explode, or burn too quickly.
Further, as fuel, pour 4.7% sulfur, as well as 11% ordinary gelatin, into the mixture, which will play the role of both fuel and glue.
As a combustion catalyst, you still need to add 1% potassium dichromate to the mixture, as well as 1% sodium alginate to improve the viscosity of the mixture.
Now we add water and begin to gradually mix the main substances so that they become a homogeneous mass.
After everything has dissolved, we add to the mixture the most important chemical - potassium chlorate, which plays the role of a powerful oxidizing agent, that is, a substance that makes the mixture burn.
Now all this is mixed again until smooth. Then water is added to achieve the desired viscosity, and basically everything. It remains only to apply this mass to the tip of the match.
To impart color to sulfuric masa, part of the sand can be replaced with iron oxide or other inert dyes. While the matches are drying, it remains to do another important part - the matchbox itself and the grater surface, on which the matches will light.
The author has already made the boxes in advance by simply gluing the pieces of cardboard, by analogy with ordinary matchboxes.
To create a grater surface, a mixture of red phosphorus and other fillers in the form of the same sand, antimony sulfide and other reagents is used. But the author did it simply, did not stint on phosphorus and mixed it with PVA glue.
Then I smeared this mixture on the ribs box.
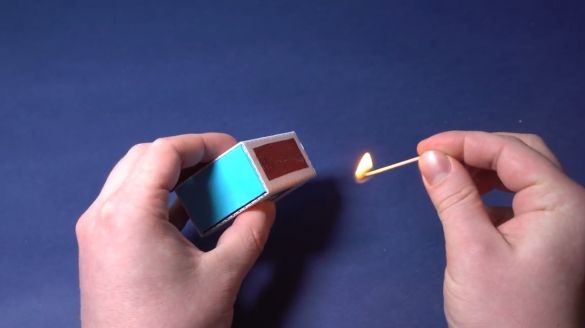
After the mixture has dried, the grater surface is ready. By the way, the matches have also dried up, so you can collect such an impromptu matchbox.
The author decided to brand these matches and named them “Thoisoiiki”.
After everything is assembled - the moment of truth comes. Let's check whether such a makeshift match against such an improvised box is lit.
She is on fire. Amazing As you can see, home-made matches were no worse than the purchased ones. The chemical reactions involved in this process are quite simple. First, when the match head is rubbed against the surface of red phosphorus, together the contact of potassium chlorate actively oxidizes red phosphorus. And from this temperature begins the reaction of sulfur and potassium chlorate in the head of the match. Then gelatin is already reacting. The resulting heat boils paraffin, which is impregnated with a match.Then he lights up, setting fire to the wooden straw itself.
Now, let’s compare with a microscope homemade matches and those made at the factory.
It can be seen that the structures of both matches are very similar. Even in factory matches there are air bubbles that, although not very much, still worsen the burning of the match. From this we can conclude that the manufacture of matches is not such a complicated process. However, for more than 100 years, humanity has faithfully used these tools to receive fire. By the way, in some countries you can still find dangerous matches mentioned in the beginning of the article on sale.
For example, in England and in the USA, one can easily find those matches that can easily be ignited by friction on almost any surface.
As you can see, matches familiar to all are not as simple as they seem. But nevertheless, the author does not advise to engage in their independent production, due to security reasons.
Thank you for attention. See you soon!
Video: