
Simple technical experiments are very useful to do with children, this is spending time together, instilling skills, and understanding by small designers, the basics, that the loaves, as in the famous cartoon, do not grow on trees.
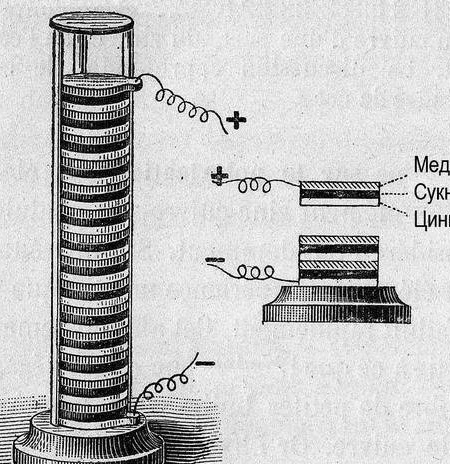
This time, we decided to make the simplest chemical source of current, and try to apply it for anything practical. Speaking about the practical application, it is worth recalling that just a few generations ago, radio amateurs, to supply their battery radio receivers and amplifiers, were offered to manufacture several types of galvanic cells or batteries for independent production. These are the elements of Leklanshe and Popov [1] p. 9 ... 18, or a lead-potash or gas battery [1], p. 22 ... 28. Several, relatively high-current elements were connected to an incandescent battery (incandescent filament of radio tubes), dozens of smaller elements, to an anode battery, the voltage of which could reach 60-80 volts. The batteries were “wet” - with liquid electrolyte and required care and maintenance.
So, galvanic cell, a few words “how?” and why?". Electric current occurs when metals interact. In this case, a different potential difference (voltage) occurs. Back in 1793, Alessandro Volta, constructing a galvanic cell (Volta pole), established the relative activity of the then known metals: Zn, Pb, Sn, Fe, Cu, Ag, Au. The "strength" of the galvanic cell turned out to be the greater, the farther apart the metals in this row stood (the series of voltages).

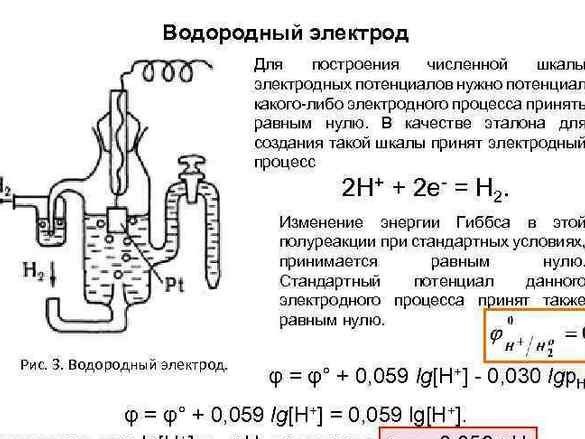
Later, to organize the data, the potential of the “hydrogen electrode” was taken for zero reference. After measuring the potential of the metals paired with him, the experimental metals were arranged in a row. The resulting table was called the "Electrochemical Series of Metal Stresses" and in the chemistry cabinet, must hang next to the periodic system and the portrait of Dmitry Ivanovich.
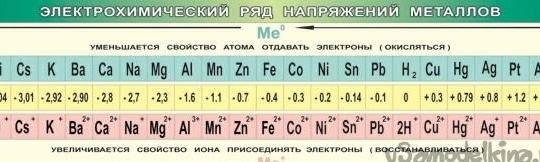
A number of metal stresses are a useful contraption, in our case, we, like Alessandro Volta, will know - the further the metals are separated from each other, the greater the tension will be obtained.
In our experiments, like the classics, we used copper and zinc.When the plates are immersed in an electrolyte, between it and the zinc plate, a chemical reaction occurs, as a result of which negative charges accumulate on the plate and it is negatively charged. As a result of the reaction taking place in the galvanic cell, the zinc electrode gradually dissolves.
On a copper electrode, during the operation of a galvanic cell, tiny hydrogen bubbles are formed that isolate the surface of copper from the electrolyte. The phenomenon is called, in a galvanic cell it is harmful, they are struggling with it. To remove the released hydrogen, substances called hydrogen are introduced into the electrolyte. In their role are often compounds of manganese, copper sulfate. In simple experiments, pharmacy potassium permanganate can be used.
What we used for the experiment.
Devices and materials.
For the assembly of galvanic cells, as copper electrodes, you can use wire, wire, foil. Zinc can be extracted from dry elements, galvanized products can be used. Instead of zinc, you can try to use an electrode of aluminum or iron. Sodium chloride for electrolyte, a bit of soft mounting wire. You definitely need a voltmeter or multimeter, wire cutters, scissors. As vessels, non-metallic containers of a suitable size can be used. Glass, more convenient than light plastic cups - they are heavier, more stable, more difficult to knock over. It is very good if there is a low-current low-voltage load - a simple radio, a quartz watch, etc.
"High voltage" battery of wire and screws.
Fascinated by the simplicity of the details, and the relatively high voltage received, we tried to assemble such a battery. A “classic” pair of metals — copper-zinc — is used here. The idea is to use galvanized fasteners as a zinc electrode. Gracefully. It is clear that such an element is not designed for long-term operation - a thin layer of zinc will quickly dissolve, however, this is not important for a short-term experiment. But galvanized screws or cogs are everywhere full.
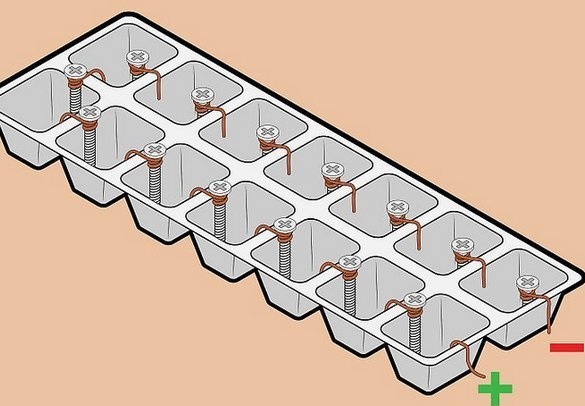
A wire is also used as a copper electrode - also a widely available material, in addition - the most convenient installation of elements in a battery - all elements are connected in series - plus one to the minus of the next. In this case, the voltage is summed, the current remains the same.
Let's get started.
After selecting the required number of galvanized fasteners of the desired length, we found a suitable copper wire. This is a winding wire in varnish insulation. The diameter of the wire is about 0.5 mm.

Pole cleans the varnish insulation, several times, with effort pulling the wire through a double-folded grinding skin of medium size.


Then, he prepares a pair of electrodes - under the head of a self-tapping screw, tightly wraps two or three turns of wire and cuts off the excess.

Battery assembly - a trough was used as a container for freezing ice. You can use cells from boxed sweets, however, they are more subtle. After installing the electrodes on the walls between the cells, we fill the containers with electrolyte. We used a solution of table salt - a tablespoon with a slide of 0.5 liters of warm water. For filling it is very convenient to use a medical syringe.


We found a few more screws for the electrodes and added elements to the battery, that’s what we got. The voltage at a high-impedance load (input resistance of a digital voltmeter) is considerable, but at any load, however noticeable, it drops significantly.
Try to do similar galvanic cell (battery) with larger electrodes.
As a container, we used a half-liter jar (two), plates of a considerable area will fit in it. As electrodes, we took thin copper foil and zinc - the remnants of a glass from a factory "dry" element, disassembled during the production of graphite for refractory coating.

We cleaned the remnants of dried crystalline salts with a wire brush and cut two plates with approximately scissors with approximately the same area. From copper foil, cut two matching stripes. Also with scissors. We got two pairs of electrodes, which equipped our elements, without further ado, bending their edges on the neck of the can.


In a larger container, we prepared an electrolyte - sodium chloride, dissolved in warm water, the concentration is the same, and prepared elements are poured.


We connected the two elements in series, using a piece of the mounting wire and two crocodile clips. So, fine, the battery voltage is close to the standard "finger", try to use. One element with a voltage of 1.5 V is used in electromechanical watches, in addition, the current consumption of the watch is very small and our battery will be able to overpower it.

We removed the standard battery from the watch and connected to the terminals a piece of the mounting wire. Observing the polarity (copper plate - "+", zinc - "-"), connected our watch to a makeshift battery, voila! The clock works, the voltage "sinks" to 1.3 V. The clock worked perfectly for several hours, until we all boasted (however a sorcerer!) Then we got tired.

To the track.
The internal constitution of any child is such that attention on one subject, he is able to focus no more than 15 ... 20 minutes, and all classes with children should be planned so that they fit in at that time, or switch between different classes, otherwise you will both be tormented.
As a load, it’s better to apply that whether it’s moving or luminous - the numbers on the voltmeter impress the mind, but not the heart. In addition to watches and calculators, it will certainly cause admiration, the work from a home-made battery of a small radio receiver (as an option - home-made!).
For long-term use, the electrolyte of the cells should be protected from dust and evaporation, and take care of the depolarizer - well, at least clogging the jar with a piece of plastic film with an elastic band and adding potassium permanganate to the electrolyte. Moreover, it is better to immediately collect the mentioned element of Popov.
In addition to galvanized self-tapping screws, it is possible to use galvanized sheet steel, for large elements it is more convenient - during the experiment, you can get a significant current and power whatever (moving your fingers in the air).
Bibliography.
1. P. Strelkov. Know and be able. Pioneer electrical engineer. Detgiz. 1960 year
2. V.S. Polosin, V.G. Prokopenko. Workshop on the methodology of teaching chemistry. Moscow, "Enlightenment", 1989, pp. 202,203.

