Hello dear the inhabitants of our site and readers.
A simple Italian guy, the author of the YouTube channel, will challenge nature itself and try to create in a few minutes what it has been creating for millions of years. Well, as you understand from the name, it will be an artificial ruby. The author does not mean that he will take some materials, give them the shape of a ruby and then paint them, no. The author will take the chemical elements that make up the natural stone, and using them will create his own, artificial ruby.
I suggest that you familiarize yourself with the manufacturing process by reading this article or watching a video.
By the way, this article is provided for informational purposes only. I highly recommend not repeating such a process on my own, in view of the danger thereof.
Tools and materials used by the author.
Manufacturing process.
The process of preparing chemical elements will be quite dangerous, so the author puts on a respirator and safety glasses.
At the very beginning, the author takes a glass container with a volume of about 1 liter and pours a solution of hydrochloric acid 31% into it.
He needs to dissolve aluminum in acid; for this, the author places small pieces of aluminum foil in a vessel.
The author carefully soaks the foil in acid with a stick, a rather violent chemical reaction takes place during dissolution, with the release of toxic vapor.
Aluminum is completely dissolved, the author leaves the container for 12 hours.
After 12 hours, the solution changed its color.
Now the author adds sodium bicarbonate (baking soda) to the jar to quench the acid. In the process of quenching the acid, sediment falls to the bottom.
The author introduces a significant amount of distilled water into the solution, it dilutes the acid but does not dissolve the precipitate.
There is a white precipitate at the bottom of the can, so the author needs it.
The author pumps out the already unnecessary fluid with a syringe.
The sediment must also be removed from the liquid. To do this, the author dried it in the oven.
The output was a fine white powder, this is aluminum oxide (Al2O3) Just what the author needs.Indeed, according to the site, ruby consists of aluminum oxide.
But that's not all, now the author adds chromium oxide (Cr2O3), which the author did not have to produce, such a powder can be simply
Chromium oxide is often used as a pigment for green paints. And also it is used in jewelry, by the way GOI paste for 60-70% consists of this substance. In the video, the author was apparently mistaken in indicating the chemical formula of CrO, which is also chromium oxide but has a black color.
Let's continue, the author adds two small portions of chromium oxide (green powder) weighing about, to alumina (white powder) For every 100 g of alumina, 0.52 g of chromium oxide is needed, according to the author, but it seems to me that he poured more. In general, the content of chromium oxide should not exceed 2%. Mixes powders until a uniform color is formed.
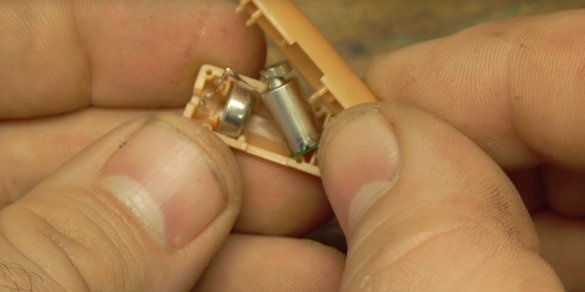
Now the author needs to make a device that will provide the supply of powder in small quantities and with equal speed. To do this, he extracted two of some objects that, as it turned out, were toys for cats. The same motors are present in every mobile phone.
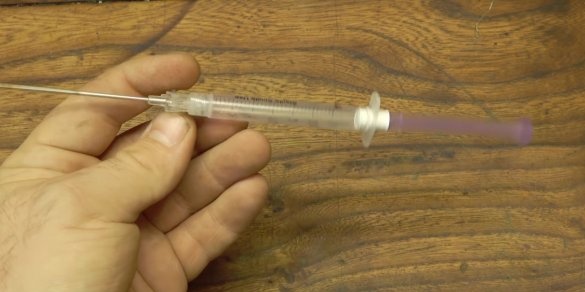
The author soldered the wires to the contacts of the motors. The author himself glued motors to hot melt adhesive to the syringe body. The author pulled the piston of the syringe, it will no longer be useful.
The idea is that you need to place a portion of the powder in the syringe, and when the motors are turned on, a vibration will be created that will ensure uniform flow of the powder through the needle.
The author will connect the motors to a 3V battery.
For verification, the author downloaded the first batch of powder, and connected his device to power. Apparently it works, the needle vibrates, but there is no desired effect, the powder practically does not spill. The needle was too thin.
The author decided to replace the needle with a thicker needle for the pump. One motor attached directly to the surface of the needle, but again there is no desired effect.
Now the author takes a piece of a thin copper tube, mounts the motors again. Checks, this time everything worked out, the mixture pours evenly. You can start making ruby.
For this step, the author will need a flame with a high temperature. He will use his hydrogen burner, which I wrote about in the previous article.
The author places a portion of the powder in his feeder, turns on the vibration. By the way, the author himself holds the device over a refractory stone. Further brings the flame of the burner, the flame temperature should not be lower than 2000 ° C and melts the precipitated powder. The idea is that every grain of sand should melt before it falls to the previous one.
The flame of the burner is so hot that the author's heat-resistant material does not withstand. So he puts the debris aside. And it will repeat the process on the surface of graphite, which can withstand very high temperatures.
The author repeats the procedure and apparently repeatedly. This time the author succeeds. The grains of sand in the stream melt and begin to crystallize.
After a drop has formed, the author continues to hold the burner flame over it for another two minutes. It seems that the author still manages to get his first ruby crystal.
As it cools, the crystal gradually changes color, and eventually acquires a red tint.
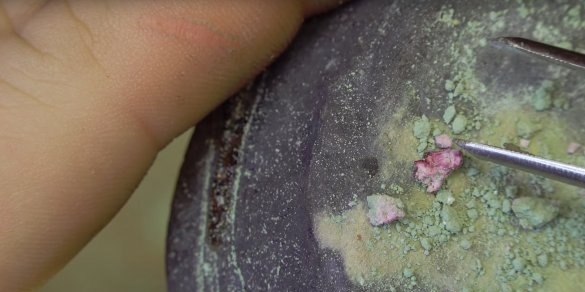
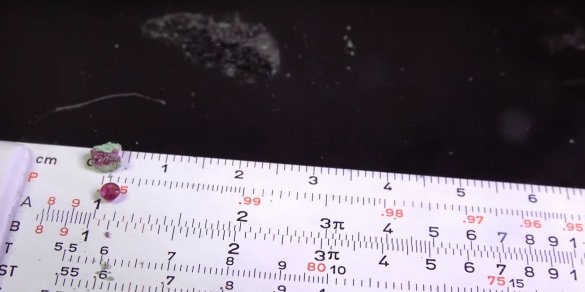
The author managed to make several samples of the mineral. The largest instance, reached a size of almost 5 mm.
It seems of course that he is not big, but the author compares it with a sample that he temporarily removed from his mother, and it turned out that his sample is much larger. Of course, this is only a raw form which, after processing, will significantly decrease in size.
The author checks the ruby with ultraviolet light. As you know, genuine rubies, under the influence of ultraviolet radiation with a wavelength of emitted light of about 365 nm, give uniform red fluorescence. We look at the results in the photo.
On this, I say goodbye to you, thank you for reading. Good mood to all, bye !!!