Hi everyone, I am starting to conduct a series of experiments that I have long wanted to do. Specifically, this article will be devoted to the experiment with a liquid conductor and the creation of a rheostat based on it. Such a rheostat can control a variety of capacities, from a few watts to several hundred or even thousands of kilowatts, however, in the latter case, the dimensions of the rheostat will be very rather large. But in general, I am not interested in a rheostat, I am interested in the properties of liquid conductors, in my case it is ordinary water with a conductor in the form of kitchen salt. So, let's get down to business.
Materials and tools that were needed:
Material List:
- table salt and water;
- wire (I have copper);
- boards;
- a bolt, a nut (and another piece of something for the handle);
- self-tapping screws;
- Super glue;
- a piece of soft tube;
- wires, power supply, LED or other load.
Tool List:
- a hacksaw;
- ;
- screwdriver;
- ;
- .
Manufacturing process:
Step one. The foundation
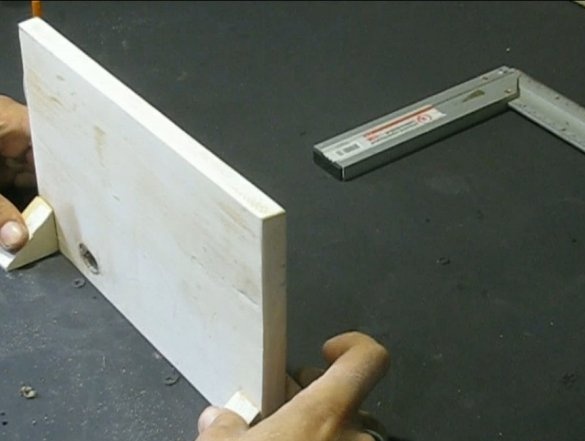
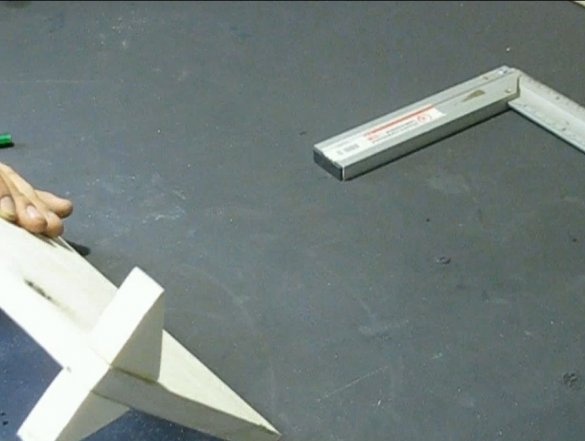
He riveted the base for an ambulance from the boards, everything can be glued with superglue or twisted with screws. You can make a base from other materials, for example, from wire.
Step Two Valve
By clamping the tube, we reduce the cross section of the liquid conductor, as a result, less current passes through it. Of course, it is more convenient to use a faucet here, but it should be made of plastic or other material that does not conduct current. However, my design works well, and most importantly, clearly.
The clamp was made of two bars, glued a nut in the upper one, and sharpened the bolt that was twisted into it at the end. A stud was welded to the head of the bolt as a handle. At first I wanted to make the pressing part of wood, but everything worked tightly, as a result I took a coin, it has a recess in which the end of the bolt enters. Here are such mini-vices. I screwed the pieces of the bars with screws.
Step Three A tube
We install the tube, I attached it with wire brackets. We install electrodes in the tube on both sides, in my case it is a copper wire. Of course, copper from salt and electrolysis quickly collapses, but did not want to mess with stainless steel, and for the sake of the experiment, there will be enough copper.
The ends of the electrodes are inserted into the holes in the board and glued, sit tight. In the end, you can pour and electrolyte, in my case it is water with a high salt content. I added ink from the printer as a dye. That's all, now solder the wires, looking for a power source and load.
Step Four The experiments
1. As an experiment, I connected a 12V / 4W lamp, I did not pull the rheostat, and electrolysis started. The point is the small area of the electrodes, it is not designed for such power and more than it can, the rheostat will not allow current.
2. I connected the LED from the flashlight, I do not know how much Volt and Watt it is, but the 9V crown does not glow at all its power. The rheostat controls the LED perfectly, there is no electrolysis, or maybe it is too weak, and I do not see it. It is not so easy to completely turn off the LED with a rheostat, you need to tighten the tube very much to displace all the water from it.
3. I connected the motor from the drive together with the LED, the rheostat controls perfectly the revolutions, and the brightness of the LED has become much easier to adjust, the adjustment range has become smaller. The fact is that the motor is able to operate at a lower voltage than the LED. While the motor reduces speed, the LED is already off.
As for electrolysis, with such a load it proceeds, but not very actively.
conclusions
The rheostat is viable, its power depends on the area of the electrodes, and the operating voltage depends on the length of the tube (liquid conductor). The farther the electrodes are from each other, the less conductivity between them and the greater the voltage required.
Of course, the lack of a rheostat is in the evolution of gas and heating of the liquid, but, as I said, the idea is not to create a rheostat at all. At the moment, I am interested in what will happen with a large current on the thinnest section of a liquid conductor. So, the wire simply burns out, and water can decompose into hydrogen and oxygen. Of course, experience has not yet confirmed this, and it will not confirm it, probably, because with a decrease in the cross section the current strength decreases, which is necessary for the splitting of water into oxygen and hydrogen. But in this case, you can try to increase the voltage ...
If you have ideas what else to check with such a rheostat, write, we will conduct an experiment!