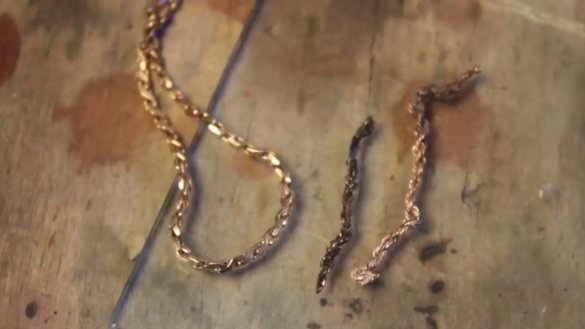Greetings the inhabitants of our site!
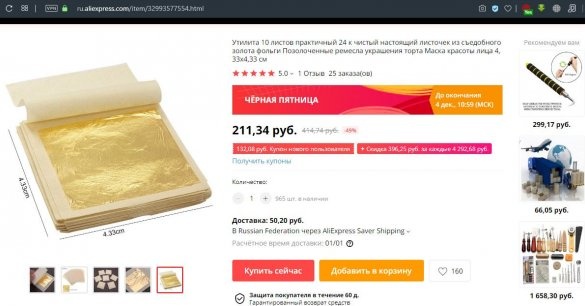
This article is about gold. The story is this: the author of the YouTube channel "Fiery TV" decided buy gold on ... Aliexpress.
The first thing that comes to mind is what kind of gold can be on Aliexpress. But if you look closely and read reviews about the product, it becomes clear that it seems to be quite a real gold leaf 24 carats.
Well, in general, to get rid of doubts, the author still ordered it and the package was delivered, now we’ll check whether it is really possible on Aliexpress order real gold and take him across the border, well, at the same time check homework ways to test gold and its test.
So, the wedding ring 585 of test will undergo tests:
Here is such a gold chain, forged by masters in the 90s from dental crowns, cigarette foil and something else:
When the evaluator checked this chain, he said that here he did not even reach the 500th test.
Next, check a small piece of a certain red chain very similar to gold:
Let's check the sample of the “golden” brass fitting:
Test the reagents on a bronze product:
Well, we’ll check the gold leaf ordered on Aliexpress by itself:
So let's get started. The first and most popular method, which was found on the Internet - is to rub a suspicious yellow product on the bottom of a ceramic mug.
A golden strip should remain on the rough surface of the ceramics (this is in case the product really contains gold), and if the product is not gold, then the strip in this case should turn out not at all golden, but dark gray, well, in any case, it says "legend".
But as we can see, even a brass fitting leaves shining golden traces on the ceramics. And frankly speaking, bronze is not far behind. Perhaps somewhere in the parallel universe this method really works, but the author did not fix this in his workshop.
Next way verification of a gold coin for authenticity is the processing of it with an ammonia solution. The essence of the method is based on the fact that copper and copper alloys react with ammonia solutions, while ammonia turns bright blue (copper ammonia).
A copper wire immersed in a glass with liquid ammonia begins to react slowly, turning the liquid blue, gold items made of gold can be in this solution for any length of time.
As expected, the liquid in the glass with copper acquired a bluish tint.
In a similar way, ammonia acts on a brass product, instantly dissolving the oxide film on the surface of the metal and forms blue copper ammonia:
This indicates that unfortunately there is no gold in the brass fitting. Black stains appear on the fitting itself, and after drying, a light blue coating appears.
Now let's repeat the experiment, but with bronze. The author melted this instance himself and confidently states that it contains 80% copper and 20% tin.
And so what we observe: cotton wool moistened with a solution of ammonia does not turn blue, it becomes dirty gray, and the surface of bronze does not change in any way. It seems that on the contrary she begins to shine even stronger. The following conclusion can be drawn from this: rubbing with ammonia can help distinguish bronze from brass.
Then try to wipe the little gold:
After wiping the ring, the color of the cotton wool has not changed, so everything is fine. By the way, copper is also present in the jewelry alloy of gold, but it does not react.
The chain of the 500th sample also does not stain cotton and an unknown fragment of the red chain also did not affect the color of the cotton.
Another interesting way - this is to check the gold with vinegar, supposedly products made not of gold should darken in vinegar. Let's check it out. We take acetic acid (70%).
In this case, no changes were noticed. The method does not work.
Next way - applying silver nitrate in the form of a lapis pencil.
Upon contact of a wet pencil with a more active metal than silver, a dark spot should appear on the surface of the product, consisting of the finest silver powder.
As you can see, brass reacts instantly, but bronze with a high tin content is already more stable, but you need to give it time to notice some reaction:
Gold items do not react in any way with a lapis pencil (gold leaf with Aliexpress too), but the copper wire darkens when it comes in contact with silver nitrate:
AND the toughest of all artisanal methods - verification using a solution of pharmaceutical iodine.
Highly active iodine instantly begins to react with copper. If iodine is added to brass or bronze, the same effect is observed, but much more slowly.
When iodine is applied to gold products, after not prolonged contact, the gold darkens and acquires slightly iridescent stains.
By such iridescent tones one can understand that this is still a golden item and it’s time to bring it to a jeweler for polishing, because you will definitely not wipe such a stain, especially from a chain.
The unknown red chain has also darkened, like other gold items, but there are only iridescent reflections.
The purest Chinese gold has also been tested with iodine, it does not react with iodine at all, and no matter how much the author drops iodine on pieces of gold, it has not darkened.
So it's time to move from grandmother's methods to more professional ones and determine what kind of sample these golden leaves are. Nitric acid (approximately 70%) will help us in the determination.
An acid of this concentration is used to determine the gold of the 500th sample. If you put such acid on brass, then a violent reaction will occur and it will immediately become clear that gold does not smell here (although gold does not smell at all).
Bronze also reacts quite actively to nitric acid:
We turn to the verification of gold items:
The gold chain of the 500th sample lay in acid for about a minute without any signs of dissolution, but the red chain without a sample was severely affected by the action of the acid.
We can conclude that the chain without identification marks has a test below the 500th, and most likely much lower.
Now we prepare a solution for the 585th test. A little nitric acid, a little distilled water and a couple of drops of hydrochloric acid.
Immersion in acid did not affect the color of the metal, and nowhere is the transition from one color to another visible.See these and other interesting experiments in the original Author’s video:
Next, the author prepared a solution for the 958th test of gold. The solution turned out to be bright orange, which, as it were, hints at its vigor.
Drop on gold leaf.
A drop of acid touches the gold leaf and instantly burns through it the smallest pores through which it wets the sheet of paper that is under it. In general, this gold does not hold 23 carats, we will make a solution for the 900th test.
This time the dark spots are much smaller and almost invisible, but this gold certainly does not hold 22 carats and most likely has a purity of 21 carats.
Well, we figured it out, the chain really turned out to be about the 500th test, the ring, as it is written on the 585th test, but gold leaf, unfortunately, is not 24 carats and not even 23, 21 at best, oh, these narrow-eyed liars, well, at least they didn’t lie that gold.