Greetings the inhabitants of our site!
From year to year, oil production is becoming increasingly complex and the fuel obtained from it is becoming more and more expensive. In the EU countries, they generally threaten to stop producing gasoline engines, they want to replace all vehicles with electric cars. But lithium batteries are still far from ideal, and by the way, they are in no hurry to become ideal at all. In the best case, on a single charge of a lithium battery it will be possible to cover a distance of a maximum of 700 km, after which you will have to charge the battery for about a week, and if you use an ordinary outlet for charging, it generally takes a lot of time. And you just imagine what will happen if everyone starts constantly charging their electric cars, what huge loads on the power grid will be and how much voltage will drain. In general, the future of lithium batteries is still quite vague and every year more and more research is devoted to the search for new battery options.
As you know, the most energy-intensive metal is aluminum. Already in our time on some prototypes of aluminum batteries you can drive about 2000 km without recharging, and recharging this type of battery takes only 15 minutes, after which you can go further for about 2000 km.
Recharging aluminum batteries differs from recharging lithium-based batteries. Nevertheless, there is nothing complicated in it, you just need to insert a new aluminum, pour out the electrolyte and pour in a new electrolyte, everything is essentially the same as gasoline car, only this is an electric car, and there are no loads on the power grid. In addition, you do not need to produce a huge number of outlets with wires with a huge cross section to charge all these electric cars.
But not everything is so smooth here. Getting electricity from aluminum is not at all as easy as we would like. First, let's figure out what the principle of aluminum-air battery is.
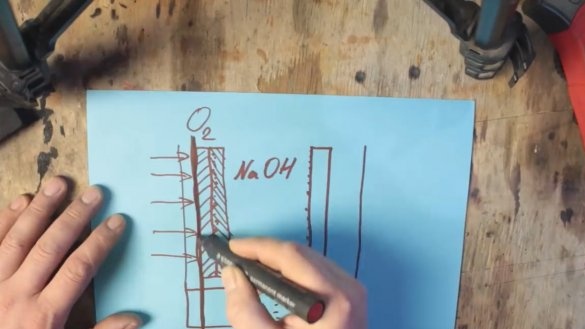
In order for such a battery to start working, 2 electrodes will be needed: one naturally from aluminum, and the second from graphite. Both of these electrodes are in an electrolyte solution.
Salt (NaCl) can be used as the electrolyte, but with it you can raise the voltage to about 0.7V. Alkaline electrolyte (NaOH) voltage can be raised already more, to about 1V.
During the chemical reaction, aluminum is coated with a layer of aluminum hydroxide (Al (OH) 3), which gradually sinks to the bottom of the tank. And on the surface of the graphite electrode hydrogen bubbles are formed, which in turn lead to an increase in resistance and a drop in voltage, this process is called polarization.
The first problem with the precipitation of aluminum hydroxide can be eliminated by simply increasing the capacity where the spent product will settle, but the second problem can be helped by a depolarizing mass based on manganese oxide, which will turn into manganese hydroxide during operation.
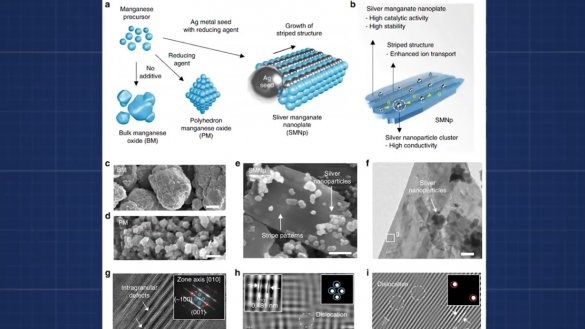
In fact, we got an ordinary alkaline battery, but only a very large one. But a new problem arises. The fact is that manganese oxide is also consumed and it will also have to be changed. And we need to ensure that only aluminum is spent. To do this, take oxygen from the surrounding air. This is where the aluminum-air battery begins. One of the walls just needs to be replaced with a gas-permeable membrane, and the graphite electrode needs to be replaced with a mixture of graphite and manganese oxide with platinum or silver nanoparticles.
Manganese oxide with noble metal nanoparticles does not react, but act as a catalyst, due to which hydrogen from the electrolyte is oxidized by oxygen in the air.
The technology for producing manganese oxide with inclusions of silver nanoparticles is not complicated in principle and can be tried in artisanal conditions. But in this article we will discuss how to make the most budget option for a battery that receives energy from aluminum. The following instructions are taken from the Fiery TV channel YouTube. In details in the original video of the author:
The most budgetary version of graphite is summer contact inserts for trolley buses. They can be found absolutely free at the final trolleybus stops, or you can buy them, they are not expensive, the author found them on sale at 22 rubles apiece.
Next, we need an alkali. Here is a tool for cleaning pipes in its composition contains one hundred percent sodium alkali.
To start the alkali reaction, we need just a little bit, 1 g of alkali per 0.5 l of water will be enough.
First of all, let's check whether a graphite electrode is really needed in this battery. For experience, let's take this stainless steel electrode.
Now we put the aluminum plate and the stainless steel electrode into the alkali, connect the multimeter and see how many volts it turns out.
As you can see, it turned out about 1.4V. Now let's check the short circuit current.
Short circuit current turned out in the region of 20mA. What conclusions can be drawn: theoretically in extreme conditions it is possible to assemble a battery of stainless steel mugs and aluminum foil.
Next we will have a copper electrode made of electrical copper.
As we can observe, the voltage turned out to be slightly higher than 1.4V, but the short-circuit current was at first high, but then it started to sag quite quickly and the copper also began to become covered with a dark coating, most likely this effect was caused by impurities in the water, since In this experiment, the author took a tap from a tap.
Now immerse the graphite electrode in the electrolyte solution.
With this electrode, a voltage of 1.3 V was obtained, the short circuit current stopped in the region of 17 mA. At first glance, it seems that the stainless steel electrode is more efficient, but the surface area of the stainless electrode is larger, so it is not yet known which graphite or stainless steel is better.
Since graphite has a fairly large resistance, you need to somehow deal with it. It is necessary to make electrodes from a material that is well conducting, and graphite should only be on its surface.It was decided to drill through the graphite, and in the resulting holes cut the thread for the m6 bolts.
The result is a steel electrode with a graphite shell.
The resistance of not drilled graphite is about 4.5 Ohms, but of drilled graphite is about 1.7 Ohms.
On the face, a decrease in resistance, and, consequently, the effectiveness of the structure will increase. In further experiments, we will use distilled water.
The first experiment with an electrolyte, in which 4 g of alkali per 1 liter of water.
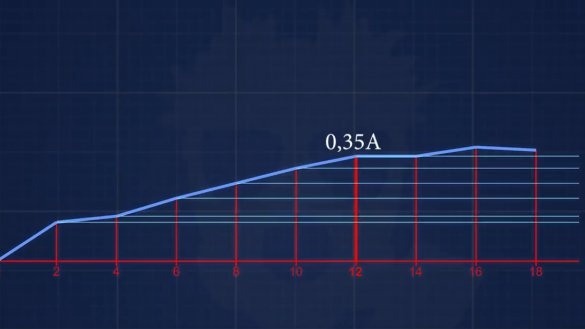
Short circuit current turned out 150mA. The next electrolyte has a concentration of 6 g of alkali per 1 liter. Well and so on, each time we will increase the concentration by 2 g until we reach a concentration at which the current will not increase.
Even though such a simple battery does not have a large current efficiency, but such a battery can work for a very long time, and any aluminum can be used as electrodes, which can easily be melted into electrodes of any shape, for example, aluminum cans various alcoholic and non-alcoholic drinks, chocolate foil, etc.
As a result, after all the experiments with different concentrations of electrolyte, it becomes clear that with this design of the battery it makes no sense to add more than 12 g of alkali to 1 liter of water, that is, we get about 1% solution.
Then the author assembled another clip, consisting of 3 electrodes.
Two batteries give a higher voltage and less loss, so the result is better.
Now, let's take a bucket of electrolyte, a large piece of aluminum and 2 stainless steel electrodes.
In a bucket, an electrolyte concentration of 10g / 1l. Peak current 1.3A, it sagged to 520mA. With all the huge area of stainless steel, it did not compare with graphite, because it turned out to be 600mA with graphite. By the way, hydrogen is released during the reaction, which can also be collected and used as an energy source. In short, there is room to grow. That's all for now. Thank you for attention. See you soon!