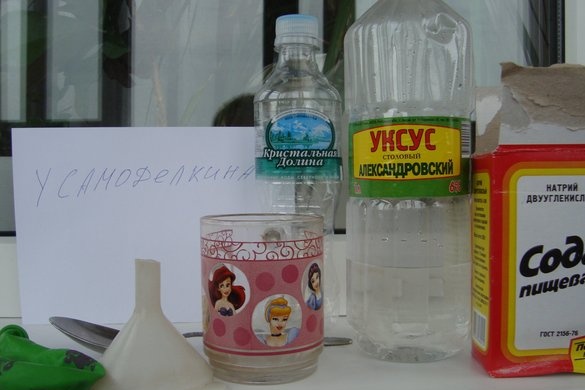Let's start experimenting! The essence of this experience is to use simple home kitchen utensils, which are well-known representatives of acid and alkali: vinegar and soda, inflate a balloon without using your own lungs or air flow. To carry out such an experimental operation, we need the following things: an empty plastic bottle, a fairly small volume (0.2-0.4 liters), a small funnel, table vinegar, preferably 9-12%, soda used for culinary baking and a balloon.
Using a funnel, pour half a glass of vinegar into the bottle. Do not rush, be careful. Remember that sprays of vinegar corrode the mucous membrane of the eye, keep the glass and the bottle at arm's length. The brushes themselves are better protected with rubber or latex gloves, especially if the student is involved in the experiment. Do not leave the young chemist face to face with the experiment.
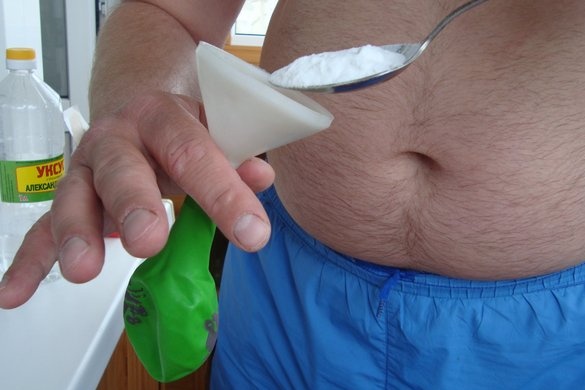
Then, with the help of the same funnel, we pour five tablespoons of soda into a ball and not into a bottle. That is, we insert a funnel at the mouth of the ball and carefully add a representative of alkali, that is, soda.
Pull the ball on the neck of the bottle. Soda begins to crumble into vinegar, begins to interact with it. A chemical acid - base reaction occurs visually, which proceeds with the release of gas. In this phenomenon, it emits carbon dioxide. It naturally rises upward, since its density is lighter than air, and abuts against the walls of the ball. The place of attachment of the ball to the neck of the bottle must be strengthened with a rope, or firmly pressed with your fingers, only with gloves on. The carbon dioxide concentration increases and the ball is inflated under gas pressure.
Simple visual chemistry will captivate every student.