The network has a huge number of different compositions and videos for the manufacture of chemical light sources. Most of them, however, are not true and completely useless. There are ingredients that are difficult to find, and even if they succeed, they cost a lot of money: for example, from 500 to 1500 rubles per 5 grams. However, it is possible to use alternative means that are cheap and it is not so difficult to find them.
As you might have guessed, we decided to devote this material to a review of a video on the manufacture of chemical light sources, but before you start working, we suggest that you familiarize yourself with this video.
So we need:
- the capacity in which the reaction will take place;
- galavit;
- hematogen in the form of butterscotch;
- ammonia;
- hydrogen peroxide purchased at a pharmacy.
As we said in the introduction, standard tools, such as aluminol, cost a lot of money, in addition, it is difficult to find. However, the author found a way to make chemical light sources, replacing expensive materials with cheap ones and slightly modifying the resulting recipe. Let's get started.
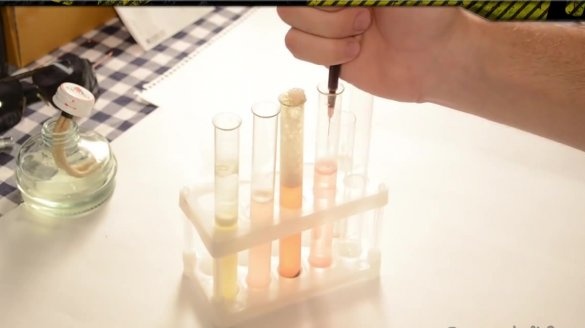
First, we need to grind a couple of tablets of galavit and pour them with 50 ml of hydrogen peroxide.
When galavit is completely dissolved in peroxide, we need to pour it in 10 ml in different containers.
The active ingredient in galavite is the sodium salt of aluminol. As an oxidizing agent we use hydrogen peroxide. The reaction takes place in an alkaline environment, so we need to add a little ammonia.
The reaction is catalytic, so we also need to add an iron ion as a catalyst. And the source of iron ion in our case is the hematogen. Cut off a small piece, throw it into the container and immediately observe the glow.
Everything is clear with the first experience. You can proceed to the second. Since ammonia smells very strong, we will use another substance - the Mole pipe cleaner. Pour a couple of milliliters of the product into the container, throw a piece of hematogen and again see the glow.
In the third experiment, the author replaces the hematogen with iron sulfate, which can be purchased in gardening stores.In this case, the reaction turns out to be very active and the glow quickly goes out.
The latest experience is not for the faint of heart, and we do not recommend repeating it. For this experiment, ordinary human blood will be needed as a catalyst. According to the author, human blood, according to the author, was the most effective.