Our experiments in the field of chemistry are continuing. This time we will again introduce you to a cognitive and bewitching experience, the result of which can amaze everyone. We will make a beautiful experience with sodium, and what exactly will happen in the end, you will find out right now.
As always, the first thing we’ll devote a few minutes to watching the video
What we need:
- 30 ml of isopropanol;
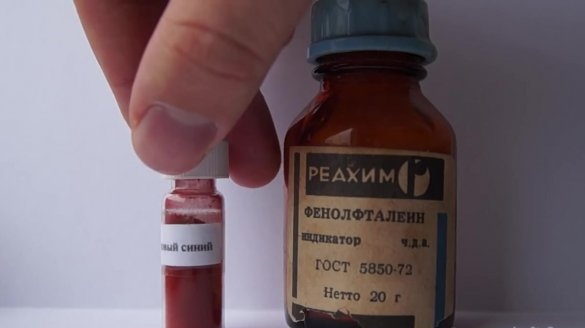
- 0.05 gr. bromothymol blue indicator;
- 0.1 gr. phenolphthalein;
- 3 drops of ethanol;
- metallic sodium
Let's start with the indicator solution, which we need to prepare at the very beginning. For its preparation, we will use approximately 30 ml of isopropanol, 0.05 gr. bromothymol blue indicator, 0.1 g. phenolphthalein and 3 drops of ethanol.
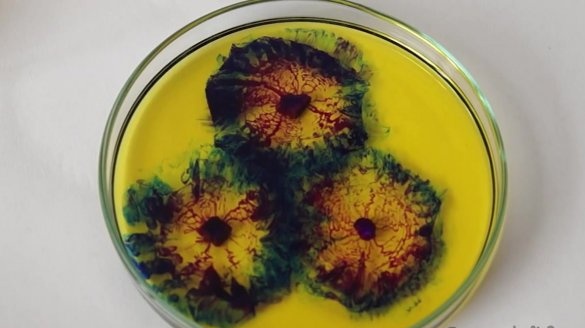
The resulting indicator mixture must be poured into a Petri dish.
For the experiment, you will also need metallic sodium, which is an active alkali metal. Take a small piece of sodium and cut it into smaller pieces.
Now let's get down to the reaction itself. Take pieces of sodium and drop them into a Petri dish with our indicator solution.
After that, you can observe a beautiful effect. Sodium reacts with isopropanol and ethanol to form an alkaline environment. The indicator responds to changes in acidity and colors the mixture in blue and purple. Bromothymol blue is accordingly colored in blue, and phenolphthalein - in raspberry. Since isopropanol has a rather high viscosity, an interesting effect occurs, such as slow diffusion of alkali, as a result of which we observe the appearance of a flower.