Humanity strives to use alternative energy. Many companies and world giants are promoting solar energy, wind energy has been actively used for many years, and so on. We decided to devote this material to a video review on such a topic. More specifically, we will produce batteries from ordinary beer cans.
We suggest starting with viewing the author’s video material
We will need:
- two beer aluminum cans;
- two graphite rods;
- crushed coal;
- Styrofoam;
- water;
- salt;
- paraffin;
- three wires.
The crushed coal can be obtained without any problems. This is the coal that remains after the fire.
The first step is to cut the top of the beer cans. This is necessary in order to more easily pour coal into jars.
In order to prevent short circuits between graphite rods and aluminum cans, care must be taken to insulate. To do this, take a piece of foam, from which we cut two washers in diameter of the cans. We put the washers in the jar.
Next, we put graphite rods in jars. Slightly push the rods into the foam so that they do not come into contact with the jars.
Now fill the cans with charcoal.
When the cans are well covered with charcoal, you need to pour slightly salted water. Salted water will be a kind of electrolyte for batteries.
It should be noted that according to the author, such home-made batteries can be easily charged.
After salt water has been poured into the batteries to seal the structure, paraffin can be poured there, which is preheated on a gas stove.
After the paraffin hardens, you can safely test the batteries.
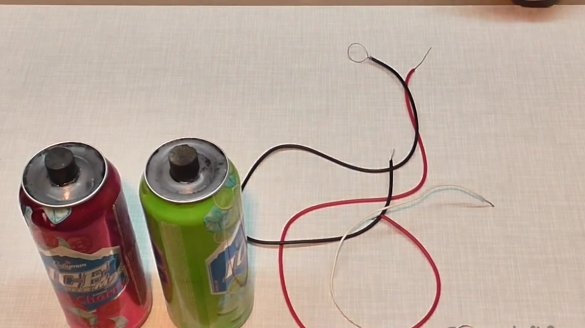
We connect the batteries in series, in order to get about 3 volts. We wind the wire on the rod of the first can and fix it with a clothespin.
Thus, it turns out that the graphite rod becomes a plus, and the jar body becomes a minus. For serial connection, we put the wire coming from the rod of the first can under the second can, so that the wire is in contact with the housing, that is, minus.
After that, we connect the second wire to the core of the second can.
We take another wire and put it under the first jar, the minus of which remained free.
Our batteries are ready. By connecting the wires to the LED bulb, you can see that it will light up.
One such battery is enough to connect the calculator, as well as the clockwork.