It happens that you find yourself in a difficult life situation, when an energy source is urgently needed. For example, you need to charge a mobile phone, turn on the radio, and so on. Elementary knowledge of physics and chemistry will make it possible to find a way out of such situations. For many, it will be interesting to know that you can "power up" the radio or charge a cell phone from an apple or lemon.

For these purposes, you will need:
- steel contact (nail, paper clip, piece of steel wire, steel coin, and so on ...);
- copper contact (copper coin, a piece of copper wire, any copper plate, etc.);
- lemon, and if an apple will be used, you need to choose as acidic as possible;
- two wiring to connect to the "battery".

Procedure:
Stage 1. We are looking for a suitable "energy source"
The easiest way is to find an apple while in a country house, village, or just lost in the forest. The best option would be a sour apple, as acid is a key component in battery life. If there is a lemon, then this is the most suitable option. You can also use oranges, kiwi and other similar fruits.

Stage 2. We establish contacts
Contacts must be inserted into a lemon or an apple, first they must be thoroughly cleaned with sandpaper, a file, or rubbed against a stone. Contacts are inserted at a distance of 2-3 centimeters from each other. The wider and longer the inserted electrodes are, the more voltage the battery will generate. If the coins act as contacts, then they must be inserted in parallel.
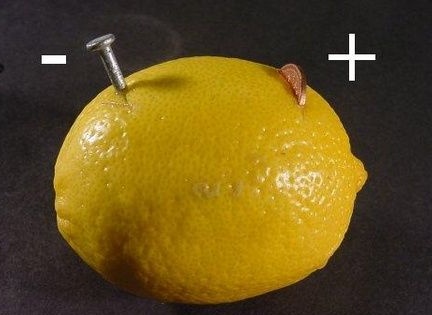
Stage 3. Connect the battery
Now it remains to connect the two wiring to the installed contacts. They can simply be gently stuck in a lemon or apple along with the contacts. That's all, the battery is ready to use. There will be a plus on the copper electrode, and minus on the steel electrode. The voltage will depend on the area of the electrodes and the acidity of the apple or lemon.


One such battery is capable of delivering about 0.5-0.8 volts. In order for a simple receiver to work or a mobile one to charge, a voltage of at least 3-5 volts is required.To get such power, you need to make several of these "batteries" and connect them in series. In our case, to obtain 3 Volts, about 5-6 such "batteries" will be needed.

Stage 4. Charge the lemons
An interesting fact is that the “batteries” created in this way can be fully charged. For these purposes, you can use the charger from a mobile phone. The author decided to use a Krona battery for these purposes.
The red positive wire connects to the copper electrode, and the black negative wire to the steel one. After charging, a voltage of 1-1.3 Volts will appear on the contacts of the "lemon".

At the heart of this phenomenon is a chemical reaction that forms between a steel and a copper electrode. The reaction itself is formed as a result of exposure to the acid contained in the lemon or apple. Thus, we can conclude that the "battery" will work until the electrodes dissolve or all the acid leaves the lemon.
