We continue the theme of chemical experiments. In this material, we will present you an overview of chemical experience using a rather interesting metal called gallium.
We recommend starting with watching the author’s video
We will need:
- gallium;
- aluminum radiator from the computer;
- aluminium foil.
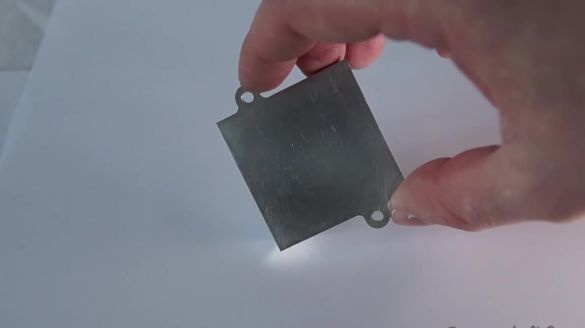
Let's start with the first experiment, in which you can see exactly how gallium creates amalgams with other metals. To do this, we take an aluminum radiator from the computer and drip about 2 grams of gallium onto it.
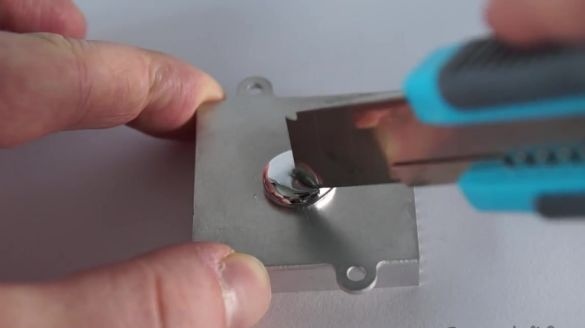
In order for gallium to react faster with aluminum, you need to scratch its surface with a clerical knife.
During amalgamation, gallium penetrates the crystal lattice of aluminum, thereby violating its structure. In this case, aluminum itself becomes very fragile like glass. To obtain the desired effect, you need to leave the aluminum radiator soaked in gallium for a couple of days.
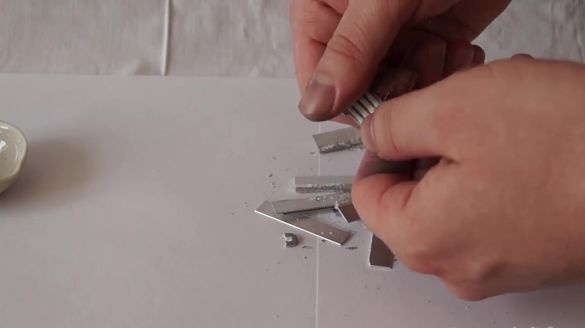
After approximately two days, the residues of unreacted gallium can be drained from aluminum. Now it’s worth a little effort to break the aluminum. If the reaction lasted longer, then aluminum would become significantly more fragile.
Let's move on to the second part of the experiment. This time we need aluminum foil.
We take a piece of foil and fold it several times. Next, cut the resulting piece into small pieces. We fill these pieces in a container and drip a few drops of liquid gallium on them.
Now you need to fuse gallium and aluminum with stirring.
Over time, you will notice that pieces of aluminum foil begin to dissolve in gallium, and aluminum foil turns into a silvery porridge. This porridge is an amalgam of aluminum and gallium.
According to the author, this amalgam has an unusual property: it should be thrown into water to observe the formation of a large amount of hydrogen. The basis of such a reaction is that during the fusion of gallium and aluminum, the first component prevents the formation of a protective oxide film on the surface of aluminum, and without this film, aluminum begins to react violently with water, forming hydrogen and aluminum oxide.
It is also worth noting that, as a result of this reaction, gallium is not consumed. It can be assembled and reused.
The author of the experience notes that this property of gallium-aluminum alloy was patented by the American company for hydrogen production, however, the project was not implemented due to the high price of gallium.